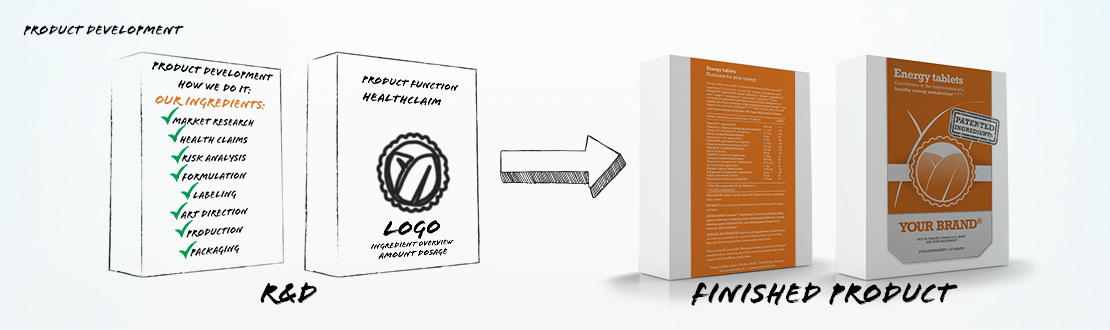
How we do it.
Regardless of the format, our health products are the result of a standardized process for research, risk analysis, formulation, design and development. The product development team collects and reacts to ideas from a variety of sources. The customer itself prioritizes development of new content based on user and business needs. Our trend watchers keep an eye on breaking health news worldwide, reading broadly and gathering periodically for product planning meetings. In other words, Sanoplus is rich in knowledge that can inspire your marketing executives with the latest developments.
